Myasthenia gravis genetic
If you tested your DNA with a personal genomics service like 23andMe, AncestryDNA, FamilyTreeDNA, MyHeritage or another testing company, you can learn more about your risk factors for hundreds of diseases. By clicking the button above ⬆️, you can upload your raw DNA data file and receive a personalized 250-page health report with research links that is the most comprehensive.
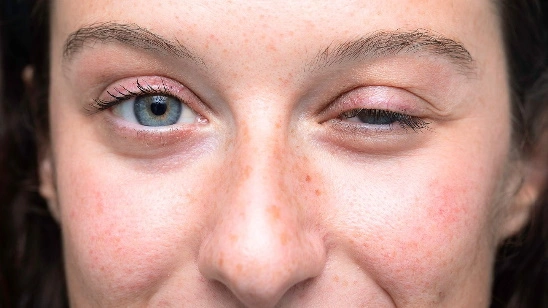
Myasthenia gravis is a condition that weakens the skeletal muscles, which are responsible for bodily movement. Typically, the weakness begins in the muscles surrounding the eyes, leading to drooping eyelids (ptosis) and difficulty coordinating eye movements, resulting in blurred or double vision. Ocular myasthenia is a subtype of the disorder where the weakness is limited to the eye muscles. However, most individuals with myasthenia gravis experience weakness in additional muscles in the face and neck. This can cause abnormal facial expressions, difficulty holding up the head, speech difficulties (dysarthria), and problems with chewing and swallowing (dysphagia), which may result in choking, gagging, or drooling.
Genetic research has primarily identified specific HLA alleles as being linked to susceptibility to MG. However, a recent GWAS study has also implicated TNFAIP3-interacting protein 1 (TNIP1) and tyrosine phosphatase non-receptor 22 (PTPN22) in the development of MG. The HLA-locus SNPs have shown a gender bias, with female-specific alleles having a higher risk for MG. This gender bias is likely influenced by sex hormones, which play a significant role in autoimmunity and MG. Therefore, further investigation into the genetic basis of gender bias in MG is crucial. Pathway-based analyses that combine information from multiple genes into a limited number of molecular networks have proven to be a powerful approach. Two pathways, regulatory T-cell (Treg) differentiation and NF-κB signaling, have been shown to be relevant to MG pathophysiology. Therefore, studies focused on these pathways may be a fruitful approach to identifying additional polymorphisms associated with myasthenia gravis.
Myasthenia gravis typically does not have a hereditary component and manifests in individuals without a familial background of the condition. However, approximately 3 to 5 percent of those affected have relatives who also suffer from myasthenia gravis or other autoimmune ailments, although the mode of inheritance remains unclear.
Follow the link of the selected polymorphism to read a brief description of how the selected polymorphism affects Myasthenia and see a list of existing studies.
SNP polymorphisms related to the topic Myasthenia:
| rs2476601 | This important SNP, located in the PTPN22 gene and also known as R620W or 1858C>T, may influence the risk of multiple autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, autoimmune thyroiditis and systemic lupus erythematosus. |
| rs3087243 | The CTLA4 allelic variant alters T cell phosphorylation patterns and causes an increased risk of autoimmune diseases. |
| rs118203994 | DOK7 mutations underlie neuromuscular junction synaptopathies. |
| rs118203995 | DOK7 mutations underlie neuromuscular junction synaptopathies. |
| rs4553808 | CTLA4 variants contribute to genetic predisposition to myasthenia gravis. |
| rs733618 | CTLA4 gene polymorphism is associated with an increased risk of developing myasthenia gravis. |
| rs121912815 | Choline acetyltransferase mutations in the CHAT gene cause myasthenic syndrome. |
| rs121912816 | Choline acetyltransferase mutations in the CHAT gene cause myasthenic syndrome. |
| rs121912817 | Choline acetyltransferase mutations in the CHAT gene cause myasthenic syndrome. |
| rs121912818 | Choline acetyltransferase mutations in the CHAT gene cause myasthenic syndrome. |
| rs16862847 | |
| rs6477872 | |
| rs772025588 | |
| rs764497513 | |
| rs794727516 | |
| rs7169523 | |
| rs6850606 | |
| rs743777 | |
About The Author
Li DaliLi Dali, a National Foundation for Outstanding Youth Fund recipient, is a researcher at the School of Life Sciences in East China Normal University. He earned his PhD in genetics from Hunan Normal University in 2007 and conducted collaborative research at Texas A&M University during his doctoral studies. Li Dali and his team have optimized and innovated gene editing technology, leading to the establishment of a world-class system for constructing gene editing disease models.